Authors
Hans Haag, BSc(Pharm) ACPR (biography, no disclosures)
Ricky Turgeon, PharmD ACPR (biography, no disclosures)
What care gaps I have noticed
Heart failure (HF) is the third leading cause of hospitalization in Canada, with a median length of stay of 7 days, and leads to readmission in 1 in 5 patients within 30 days after discharge.1,2 Iron deficiency (with or without anemia) is an important comorbidity in patients with HF, with a prevalence of 35–55% in HF outpatients and 72–83% in patients admitted for HF.3 Iron deficiency in HF is associated with worse outcomes, including increased risk of death and hospitalization, and worse quality of life (QoL) and function.3 Notably, the definition of iron deficiency used in HF is either serum ferritin <100 mcg/L, or 100–299 mcg/L plus transferrin saturation (TSat) <20%, which borrows from the definition used in chronic kidney disease.4
In addition to standard HFrEF pharmacotherapy, the Canadian Cardiovascular Society HF guidelines recommend evaluating for and correcting iron deficiency in patients with HFrEF to improve exercise tolerance and quality of life (QoL), and reduce heart failure hospitalizations (strong recommendation, moderate-quality evidence) as one of several individualized treatment options. 5,6 This recommendation was based on several meta-analyses driven by 2 randomized controlled trials (RCTs) — CONFIRM-HF and FAIR-HF — conducted in ambulatory outpatients with HF and ejection fraction ≤45%. In aggregate, the best-available evidence demonstrates that for every 100 outpatients with HF and ejection fraction ≤45% treated with intravenous iron, ~9 will avoid HF hospitalizations and 10 will have a clinically-important improvement in QoL at 3–12 months.7-11 The recently published IRONMAN trial was another prospective, randomized, open-label study in iron-deficient outpatients with an ejection fraction ≤45%.12 This study did not find a statistically significant reduction in the primary endpoint of cardiovascular death or heart failure hospitalization; however, this study had limited precision (i.e., power), high crossover in the comparator arm (17% received non-protocol intravenous iron infusions), and low use of repeat iron infusions in the intervention arm in part due to the COVID-19 pandemic.
Conversely, the IRONOUT-HF trial — the only high-quality study using oral iron supplementation — found no difference in QoL or function with oral iron polysaccharide compared with placebo. Notably, differences between iron polysaccharide and placebo in TSat (+3.3%, 95% confidence interval [CI] 1.1% to 5.4%) and ferritin (+11 mcg/L, 95% CI 0–23) were minimal after 4 months, suggesting that oral iron administration (or at least the iron polysaccharide formulation used in this trial) was ineffective at repleting iron stores in this population.13
As with use of other HF treatments, management of iron deficiency in HF is suboptimal. Access to intravenous iron products and administration (i.e., medical infusion day-bed) are major barriers to providing intravenous iron to outpatients, though this should be considered if available. Hospitalization represents a key opportunity to optimize HF pharmacotherapy, including intravenous iron. Until recently, we had no evidence to guide the use of intravenous iron in patients hospitalized with acute HF.
Data that answers this question
The AFFIRM-AHF trial, published in 2020, was a multi-center RCT of 1132 patients hospitalized for acute HF with an EF <50%, iron deficiency, and hemoglobin <150 g/L.14 Participants were ~70 years old, 95% white, ~40% with diabetes, ~50% with eGFR <60 mL/min/1.73m2, 70% had ferritin <100 mcg/L, and 82% had TSat <20%.
Over 52 weeks of follow-up, after receiving up to 4 doses of intravenous ferric carboxymaltose (shortly before discharge, and at 6, 12, and 24 weeks), the primary composite outcome of total heart failure hospitalizations (first and recurrent events) and cardiovascular death was reduced by a relative 21% (rate ratio 0.79), though this was not statistically significant (95% CI 0.62–1.01). However, in the time-to-first event analysis (a more traditional analysis used in HF RCTs), HF hospitalization or cardiovascular death occurred in 32% of patients receiving intravenous iron and 38% receiving placebo (hazard ratio (HR) 0.80, 95% CI 0.66–0.98). Intravenous iron did not reduce the risk of cardiovascular death (14% in both groups, HR 0.96, 95% CI 0.70–1.32), and all-cause death was not reported. Additionally, there was no difference in the proportion of patients with a minimally clinically important difference in quality of life from baseline to week 12–24.14 This can be explained by the fact that approximately 75% of patients in the placebo group had a minimally clinically important improvement in QoL from (in-hospital) baseline, reflecting the improvement in QoL related to recovering from their acute illness.14 There was no difference between intravenous iron and placebo in total adverse events (~64%) or study drug discontinuation (~28%).
A subsequent meta-analysis that includes AFFIRM-AHF and the aforementioned outpatient RCTs provides a confirmatory update to the best available evidence that correction of iron deficiency with intravenous iron reduces heart failure hospitalizations in patients with HF and EF <45–50%, with a number-needed-to-treat (NNT) of 14 over a median of 21 months.16 Several ongoing trials will shed more light on this intervention, particularly whether it can reduce mortality (HEART-FID [NCT03037931] and FAIR-HF2 [NCT03036462], and whether benefits extend to patients with EF >45–50% (FAIR-HFpEF [NCT03074591]).
What I recommend (practice tips)
Considering the consistent results in patients with acute and chronic HF and EF <45–50%, I have added an assessment of iron deficiency into my mental checklist for any patient with HFrEF and HFmrEF admitted for acute HF. If they meet the criteria laid out by AFFIRM-AHF, I recommend the screening of admitted HF patients for ID irrespective of anemia while admitted in hospital. Those that are iron deficient should be considered for treatment with intravenous iron depending on their iron deficiency and hemoglobin (Figure 1) while admitted to take advantage of the unrestricted IV time. Outpatients with HF should similarly be screened for iron deficiency, and receive IV iron if meeting these criteria. In patients without iron deficiency, and in those who have received IV iron, consider screening with ferritin and TSat every 4–6 months, mimicking IRONMAN and the ongoing HEART-FID trials approaches.
Re-hospitalizations within a month post-discharge from a HF hospitalization are common, and inpatient correction of iron deficiency represents a simple intervention to keep patients out of hospital.
Figure 1. Suggested iron deficiency assessment in newly admitted patients with acute HF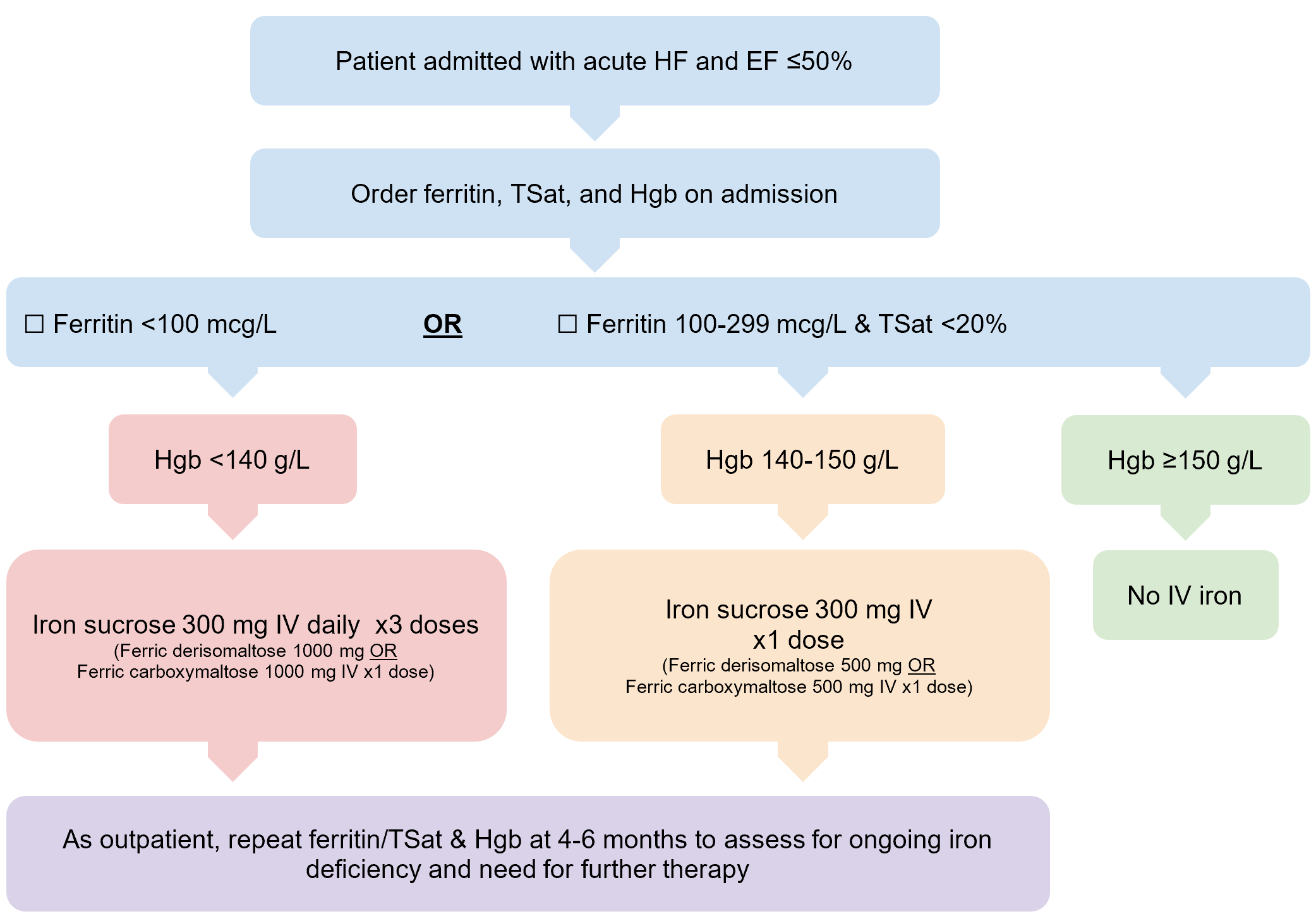
References
- Canadian Institute for Health Information. Hospital stays in Canada. 2022. Accessed April 18, 2023. (View)
- Poon S, Leis B, Lambert L, et al. The state of heart failure care in Canada: minimal improvement in readmissions over time despite an increased number of evidence-based therapies. CJC Open. 2022;4(8):667-675. doi:10.1016/j.cjco.2022.04.011 (View)
- Rocha BML, Cunha GJL, Menezes Falcão LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. 2018;71(7):782-793. doi:10.1016/j.jacc.2017.12.027 (View)
- von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail. 2019;7(1):36-46. doi:10.1016/j.jchf.2018.07.015 (View)
- Ezekowitz JA, O’Meara E, McDonald MA, et al. Comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33(11):1342-1433. doi:10.1016/j.cjca.2017.08.022 (View with CPSBC or UBC)
- McDonald M, Virani S, Chan M, et al. CCS/CHFS heart failure guidelines update: defining a new pharmacologic standard of care for heart failure with reduced ejection fraction. Can J Cardiol. 2021;37(4):531-546. doi:10.1016/j.cjca.2021.01.017 (View with CPSBC or UBC)
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J. 2015;36(11):657-668. doi:10.1093/eurheartj/ehu385 (View)
- Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436-2448. doi:10.1056/NEJMoa0908355 (View)
- Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. Eur J Heart Fail. 2012;14(4):423-429. doi:10.1093/eurjhf/hfs017 (View)
- Salah HM, Savarese G, Rosano GMC, Ambrosy AP, Mentz RJ, Fudim M. Intravenous iron infusion in patients with heart failure: a systematic review and study-level meta-analysis. ESC Heart Fail. 2023;10(2):1473-1480. doi:10.1002/ehf2.14310 (View)
- Butler J, Khan MS, Friede T, et al. Health status improvement with ferric carboxymaltose in heart failure with reduced ejection fraction and iron deficiency [published correction appears in Eur J Heart Fail. 2023 Mar;25(3):443]. Eur J Heart Fail. 2022;24(5):821-832. doi:10.1002/ejhf.2478 (View)
- Kalra PR, Cleland JGF, Petrie MC, et al. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): an investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. Lancet. 2022;400(10369):2199-2209. doi:10.1016/S0140-6736(22)02083-9 (View)
- Lewis GD, Malhotra R, Hernandez AF, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial[published correction appears in JAMA. 2017 Jun 20;317(23 ):2453]. JAMA. 2017;317(19):1958-1966. doi:10.1001/jama.2017.5427 (View)
- Ponikowski P, Kirwan BA, Anker SD, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial [published correction appears in Lancet. 2021 Nov 27;398(10315):1964]. Lancet. 2020;396(10266):1895-1904. doi:10.1016/S0140-6736(20)32339-4 (View with CPSBC or UBC)
- Jankowska EA, Kirwan BA, Kosiborod M, et al. The effect of intravenous ferric carboxymaltose on health-related quality of life in iron-deficient patients with acute heart failure: the results of the AFFIRM-AHF study. Eur Heart J. 2021;42(31):3011-3020. doi:10.1093/eurheartj/ehab234 (View)
- Graham FJ, Pellicori P, Kalra PR, Ford I, Bruzzese D, Cleland JGF. Intravenous iron in patients with heart failure and iron deficiency: an updated meta-analysis. Eur J Heart Fail. 2023;25(4):528-537. doi:10.1002/ejhf.2810 (View)

Just wondering what your approach is if the ferritin is high due to an acute inflammatory response but you still suspect iron deficiency. Do you just go by the transferrin saturation in that case? Thanks
Thank you for the review.
One suggestion is to not order the ferritin and transferrin saturation in patients with a hgb >150 as they do not qualify for therapy.
Also, please provide an updated review once all cause mortality is noted There is a potential increased risk of infection with IV iron therapy, for example, which could negate some of the intended gains.
I agree with screening all anemic patients admitted to hospital for iron deficiency, or patients with normal Hb and reduced MCV and/or increased RDW. However, I don’t feel the data is adequate to support ordering ferritin, serum iron and transferrin saturation in patients with normal Hb. Did you take into account the cost of these additional lab tests and the time they require from short staffed lab facilities?
Most hospitals limit IV iron therapy because it is extremely expensive. In previous years, IV iron constituted the no. 1 medication expenditure- millions of dollars for the health authority I work at, per year. I can appreciate that IV iron reduces heart failure hospitalizations, but it is a limited resource and there’s lots of CKD patients, IBD patients, pregnant vegetarian ladies, and others who need this therapy. Most outpatients- including many who are already anemic- end up waiting weeks for their infusions.
A cost benefit analysis would be helpful. Your protocol is not practical IMHO. Hard to justify 3 doses of IV iron to every patient with CHF and normal Hb when so many of them are not optimally treated with standard heart failure meds, SGLT2 inhibitors, screened for sleep apnea, etc.
Thank you for this article, it is certainly very interesting and well researched even if I disagree with points listed above.
I agree with the overall approach and take a fairly similar approach in my practice. One concern I do have is around the safety of x3 daily doses of IV iron sucrose during a heart failure hospitalization, as there is very literature to support this. I often use Q2D dosing as has been studied in the CKD population. I appreciate the limitations of applying this new literature without access to the newer, higher dose iron formulations for inpatients in BC.
Thank you for the comments and questions.
With regards to our approach in patients with an acute inflammatory response, we would defer back to the trial inclusion criteria used by AFFIRM-AHF (ie. iron deficient defined as serum ferritin <100 ng/mL or 100 ng/mL ≤ serum ferritin ≤299 ng/mL if TSAT 1000 ng/mL) and the TSAT is low, we would consider IV iron and re-test in 3-4 months.
With regards to the comment about providing an updated review once all cause mortality is noted due to a concern with the risk of infection with IV iron, not only was the concern with infection risk not seen in the meta-analyses of IV iron in HF discussed in our article, a much larger meta-analysis on the safety of IV iron preparations in general found no increased risk of infection.
– Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter-Gvili A. The safety of intravenous iron preparations: systematic review and meta-analysis. In Mayo clinic proceedings 2015 Jan 1 (Vol. 90, No. 1, pp. 12-23). Elsevier.
Finally, with regards to the comment about the practicality and feasibility of IV iron in all HF patients, we hoped to impress upon readers the burden of heart failure hospitalizations currently has on the Canadian Healthcare system. While it’s clear that CKD patients, IBD patients, pregnant patients, and other numerous patient groups may benefit from IV iron, we feel that the data around IV iron is convincing enough to stoke discussions on the redistribution of healthcare resources given the benefits in HF patients.
Furthermore, comparing the costs of some GDMT treatments (eg. Sacubitril-Valsartan) compared to IV iron (eg. Iron Isomaltoside 2000 mg, the higher end used in the IRONMAN trial), the yearly costs would be approximately $3200 vs $1050, respectively, in drug cost alone. Of course, there are other costs associated with IV infusion therapy that have yet to be quantified in a cost-benefit analysis in Canada.
As the benefits, and risks, of IV iron become clearer through ongoing research, perhaps we can start seeing IV iron being utilized in a more effective manner for all comers.
Can you reference your algorithm pertaining to giving iron sucrose 300 mg daily x 3 doses?
Has the efficacy of oral heme iron been tested?
I appreciate your insightful article . Thank you for sharing it. I would love to connect and discuss further and use your guidance in this field , as we are launching the first high dose IV iron approved by Health Canada for HF-ID in Canada.
I look forward to hearing from you.