“A Novel Diagnostic approach to the identification of treatable causes of global developmental delay / intellectual disability”
Dr. Clara van Karnebeek (biography and disclosures)
Disclosures: Research funding / involvement in clinical trials (Ultragenyx, Shire) and sits on an Advisory Board (Sobi, Actelion, Medunik). No conflict of interest.
Dr. Sylvia Stockler (biography and disclosures)
Disclosures: Research funding / involvement in clinical trials (Biomarin, Ultragenyx, Shire) and sits on an Advisory Board (MedUnik). No conflict of interest.
Mitigating potential bias:
Recommendations are consistent with published guidelines
What I did before
Affecting 2-3% of Canadians, intellectual disability (ID) is a lifelong, devastating condition defined by deficits in cognitive functioning (IQ<70) and adaptive skills (1). It is called global developmental disability (GDD) in children less than 5 years of age; it is defined as deficits in 2 or more developmental domains (e.g., fine/gross motor skills, speech and interaction). Here we use the term ID to cover both.
In Canada, approximately 7,600-11,500 children are born annually with GDD (2). Associated co-morbidity is significant, and includes epilepsy, autism, psychiatric/behavioural disturbances, sensory deficiencies, and systemic organ involvement (e.g., congenital heart defects, liver disease).
The etiology of ID is diverse and includes infectious, traumatic and toxic origins. However, genetic etiologies are the most frequent cause, demonstrable in more than 50% of patients, ranging from numeric and structural chromosomal abnormalities and submicroscopic copy number variants, to methylation abnormalities, and single gene defects. Given the rapid development of novel genomic technologies, the number of genetic etiologies being identified is steadily increasing.
Because of the etiologic heterogeneity, diagnostic evaluation of children with ID is a challenge for neurologists, geneticists, and paediatricians.
Current guidelines for the clinical evaluation of genetic causes of ID are based on frequencies of single conditions and yield of diagnostic methods and procedures (3); cytogenetic testing from chromosome abnormalities etc. However, causal therapy is not available for many of the conditions identified by this approach, thereby limiting any potential therapeutic benefit.
Early diagnosis of individuals with genetic causes of ID that are amenable to therapy would not only mitigate the likelihood of brain damage, but also reduce the sequelae and associated health care costs. Inborn errors of metabolism (IEM) constitute a subgroup of rare genetic conditions for which an increasing number of treatments is becoming available.
What changed my practice
In 2012, we published a systematic literature review which identified 81 IEM –increased to 2014 anno 2015- that present with ID as a prominent feature and which are amenable to causal therapy e.g., medical diets, vitamins, medication, stem cell transplant (only 1 expensive drug) (4). The evidence created by our research has been translated into a two-tiered diagnostic protocol currently implemented in our institution, BC Children’s Hospital, as well as by pediatricians throughout BC, as part of a joint 18-month pilot initiative with Child Health BC in an effort to provide equitable access to the protocol for all children province-wide and evaluate its efficacy.
What I do now
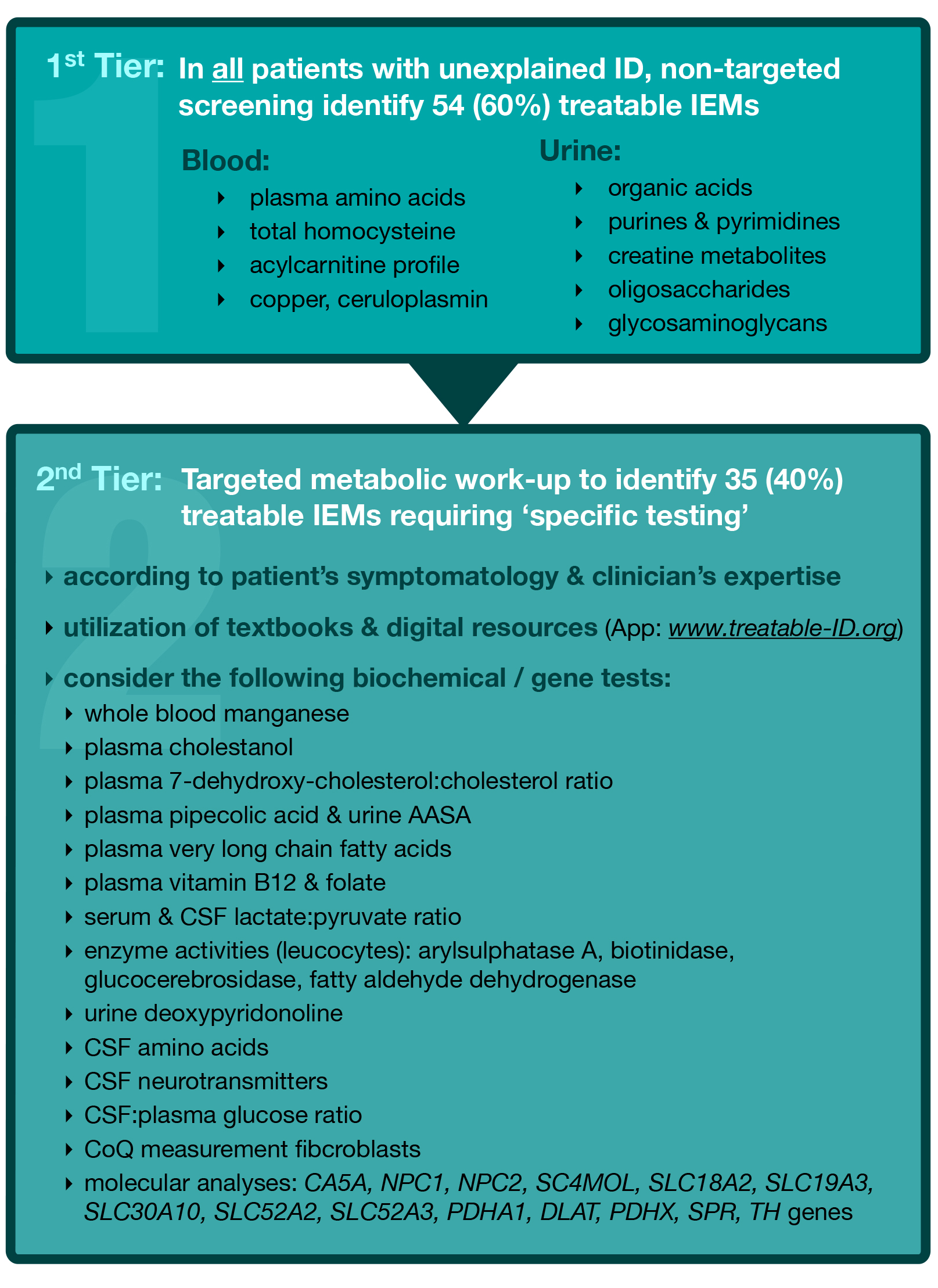
Identification of GDD or ID in children is the essential first step and often a task for the primary care practitioner, who should refer the patient to a pediatrician or subspecialist in the community or academic setting for further diagnostics. The TIDE (Treatable Intellectual Disability Endeavor) protocol is designed for pediatricians, neurologists, geneticists and other childhood specialists, to identify treatable IDs at the outset of diagnostic work-up in at-risk patients, and was published as Recommendations in Molecular Genetics and Metabolism, (2014) (5). The first tier involves biochemical group tests, which potentially indicate 60% of the currently known treatable IDs. [Figure 1]. This first tier can and should be applied in all patients with unexplained ID, by community paediatricians and specialists, to effectively exclude a majority of treatable IEMs without referral to a specialised centre. Generally, these tests are provided by most biochemical genetics laboratories across Canada, with reasonable turnaround times and affordable prices. In BC, these tests are funded by the medical services plan for a total cost of approximately CAD $527.97 per patient (5).
If so indicated the next step usually performed in an academic setting, is to apply the current diagnostic practice parameters for ID, as suggested by the American Academy of Pediatrics 2014 (e.g. chromosome micro-array, single gene tests, MRI, EEG etc.,) as standard diagnostic work-up(3), in combination with the second tier of the TIDE algorithm, for identification of the remaining 35 treatable IDs. These conditions require a ‘single test per single disease’ approach, including single metabolite or primary molecular analysis, which is designated as the second tier.
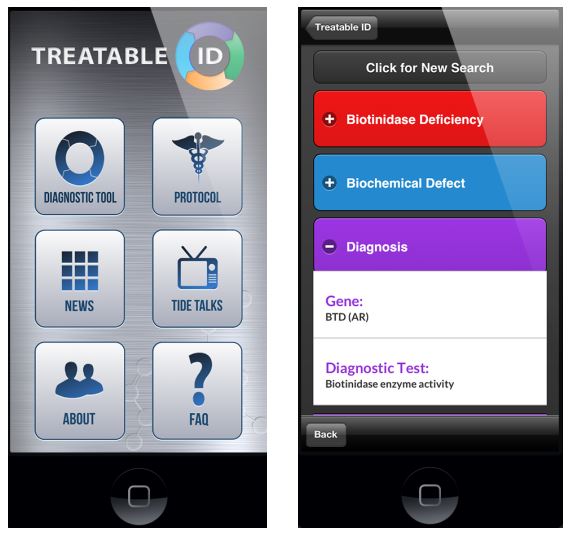
The protocol is supported by a corresponding interactive website (www.treatable-id.org) and a freely available Treatable ID App (via www.treatable-id.org and the Apple App store as TIDE-BC App) (6). [Figures 2 and 3].
With respect to treatment, our literature search identified 91 causal therapies. An overview of all therapies for each IEM is beyond the scope of this article, however a complete list of identified treatments together with corresponding evidence levels and therapeutic effects, is available at: http://www.treatable-id.org
Throughout this diagnostic process, immediate referral to an academic specialist is required according to the criteria on our website ( www.tidebc.org site), which include: abnormal 1sttier test results, neurodegeneration, developmental regression / plateauing, significant behavioral deterioration, unexplained death of a sibling, coarse facial features, hepato/splenomegaly, bone dysostoses, refractory seizures, unexplained movement disorder, MRI/S brain with unexplained abnormalities, recurrent / unexplained vomiting, documented episodes of hypoglycemia, metabolic acidosis, ketonuria,
Since the literature search was published early 2012, new ‘treatable IDs’ and new treatments for known IDs have emerged as a result of gene discovery and/or clinical research, including but not limited to carbonic anhydrase VA deficiency (carglumic acid, sick day formula) (7) and the lysine restricted diet for Pyridoxine-Dependent Epilepsy (8).
Consequently, we will continue to modify the guidelines to incorporate these and other exciting diagnostic and therapeutic advances as necessary. Recently we published a retrospective study showing cost- and time-effectiveness when comparing the treatable ID guidelines to previous practice (200-2009). (9) Further, we will publish the results of the TIDE study end 2015 as proof of principle; preliminary results indicate treatable IEMS in more than 5% of 500 study patients presenting with unexplained ID.
To enable instant use of the results of the TIDE diagnostic protocol, see http://www.sciencedirect.com/science/article/pii/S1096719214000377
References:
- Shevell, Present conceptualization of early childhood neurodevelopmental disabilities. J. Child Neurol. 2010;25: 120–126. (Request from CPSBC or view with UBC)
- van Karnebeek CDM, Stockler S. Early Identification of Treatable Inborn Errors of Metabolism in Children in With Intellectual Disability: The TIDE Protocol in BC. Paediatrics and Child Health. 2014;19:469 71. (View)
- Moeschler J B, Shevell M, & the Committee on Genetics. Comprehensive Evaluation of the Child With Intellectual Disability or Global Developmental Delays. Pediatrics. 2014.134(3);e903-e918. DOI: 10.1542/peds.2014-1839. (View with CPSBC or UBC) [http://pediatrics.aappublications.org/content/134/3/e903.long]
- van Karnebeek CDM, Stockler S. Treatable inborn errors of metabolism causing intellectual disability: A systematic literature review. Mol. Genet. Metab. 2012; [105(3)]:3368-381. (View with CPSBC or UBC) [http://www.sciencedirect.com/science/article/pii/S1096719211006081 ]
- van Karnebeek CDM, Shevell MI, Zschocke J, Moeschler J, Stockler S. The Metabolic Evaluation of the Child with an intellectual developmental disorder: diagnostic algorithm for identification of treatable causes and new digital resource. Mol Genet Metab. 2014 Apr;111:428-438. (View with CPSBC or UBC) [http://www.sciencedirect.com/science/article/pii/S1096719214000377]
- van Karnebeek CDM, Houben R, Lafek M, Giannasi W, Stockler S. The treatable intellectual disability APP treatable-id.org: a digital tool to enhance diagnosis and care for rare diseases. Orph J Rare Dis 2012;7:47. (View )
- van Karnebeek CDM, Sly WS, Ross CJ, Salvarinova R, Yaplito-Lee J, Shyr C, Horvath G. Mitochondrial Carbonic Anhydrase VA Deficiency Resulting from CA5A Alterations Presents with Hyperammonemia in Early Childhood. Am J Hum Genet. 2014 Mar 6;94(3):453-61. doi: 10.1016/j.ajhg.2014.01.006. Epub 2014 Feb 13. (View)
- van Karnebeek CDM, Hartmann H, Jaggumantri S, Bok LA, Cheng B, Connolly M, et al. Lysine restricted diet for pyridoxine-dependent epilepsy: first evidence and future trials. Mol. Genet. Metab. 2012;107(3):335-44. (View with CPSBC or UBC) [http://www.sciencedirect.com/science/article/pii/S1096719212003447]
- Sayson B, Popurs MA, Lafek M, Berkow R, Stockler-Ipsiroglu S, van Karnebeek CD. Retrospective analysis supports algorithm as efficient diagnostic approach to treatable intellectual developmental disabilities. Mol Genet Metab. 2015;115:1-9. (View with CPSBC or UBC) [http://www.mgmjournal.com/article/S1096-7192%2815%2900065-7/pdf ]
Resources
- http://www.treatable-id.org
- http://www.tidbebc.org
- http://www.sciencedirect.com/science/article/pii/S1096719214000377
Figures
- Figure 1: Treatable Intellectual Disability Endeavour (TIDE) Protocol. The asterisk * at the 2nd tier, denotes the step where the current practice parameters for the comprehensive evaluation of ID should be implemented (American Academy of Pediatrics 2014)
- Figure 2: TIDE-BC App home page (freely downloadable via the Apple App Store), including access to the Treatable ID App (via ‘diagnostic tool’ button).
- Figure 3: Example of a ‘Disease Page’ on the Treatable ID App.
Figure 1
Figures 2 and 3

Recent Comments