Authors
Dr. Jennifer Grant (biography and disclosures)
Disclosures: Received CIHR grants for unrelated clinical trials. Mitigating potential bias: Recommendations are consistent with published guidelines (BCCDC Clinical Therapeutics for COVID). Recommendations are consistent with current practice patterns.
Dr. Alissa Wright (biography and disclosures)
Disclosures: Single session advisory board meetings for AVIR Pharma, Pendopharm, Merck and Takeda. No relationship is currently ongoing. Received funding from Shire/Takeda from being the site PI on a study of a commercial product for CMV treatment. The products were antifungals, CMV treatment or antibiotics. None were related to COVID. Received speaker fees from Merck, Roche, Sanofi Genzyme for talks. The only talk around COVID was for Sanofi Genzyme and it was specific to the MS group and around the benefits of vaccination. I was a member of the COVID therapeutics committee that created the treatment guidelines. Mitigating potential bias: Treatments or recommendations in this article are unrelated to products/services/treatments involved in disclosure statements with the exception of my participation on the COVID Therapeutics Committee. We have used the CTC guidelines (which are provincial) to draft this paper.
Jolanta Piszczek, BSc Pharm, Pharm D, MSc (EBM) (biography, no disclosures)
What I did before
Early in the COVID-19 pandemic, we knew little about risk factors for progression but noticed that severe disease is associated with a dysregulated immune response — explaining why in-hospital treatments are mostly anti-inflammatory and not antiviral medications. Early management was limited to clinical monitoring and linkage to care. We knew that risk factors like age1 and underlying health conditions — for example, diabetes, hypertension, and immunosuppression — put people at risk, while vaccination status and previous infection provided protection from severe disease. Yet, we had no direct-acting antiviral medications to stop disease progression of COVID-19.
What changed my practice
In early 2022, two key clinical trials changed the landscape. Nirmatrelvir/ritonavir (Paxlovid) given within 5 days of symptom onset offered an 89% (1% vs. 6%) reduction in hospitalization.2 Likewise, remdesivir (Veklury), given within 7 days of symptom onset, showed an 87% (1% vs. 5%) reduction in hospitalization. Both studies enrolled symptomatic, unvaccinated, previously uninfected patients with at least one risk factor for progression.
The preferred drug, nirmatrelvir/rt is given as an oral course of three pills (two nirmatrelvir and one ritonavir) twice daily for 5 days. Nirmatrelvir/rt is contraindicated in severe renal disease (eGFR <30) and, due to its inhibition of the cytochrome P450 (CYP) 3A4, has many potential drug-drug interactions. Nirmatrelvir/rt is now available at no cost at many BC pharmacies (https://www.bcpharmacy.ca/paxlovid/map) and can be prescribed by any BC physician.
If nirmatrelvir/rt is not possible, remdesivir is an intravenous alternative administered as 3 daily doses within 7 days of symptom onset. It has no significant drug-drug interactions, however, its use is limited by the availability of infusion centres with adequate nursing support for administration. For this reason, only those at highest risk of progression should be offered remdesivir. Local health authorities have different means of administering remdesivir which can be accessed here via BCCDC.
What I do now
Most of the population who are vaccinated or previously infected are at such low risk for severe disease that early therapy offers minimal benefit. However, there remain pockets of the population who are still at risk of severe disease. The BCCDC has provided excellent data showing the risk in this heat map: stratified by age, vaccine doses, and number of clinical conditions.

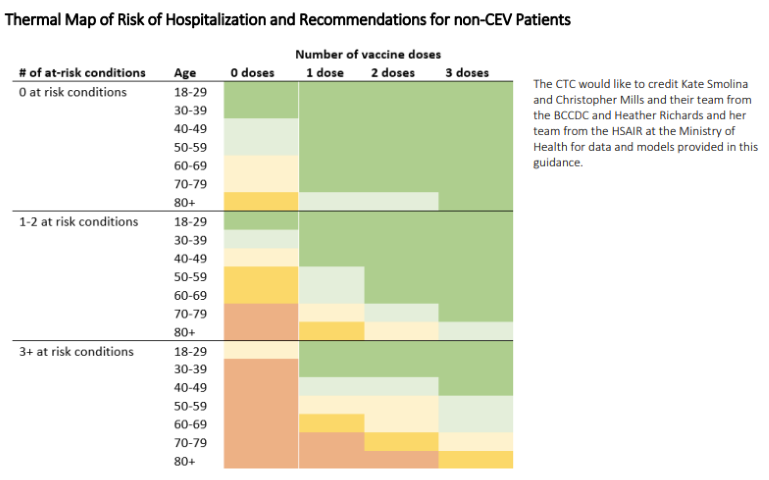
The young are at generally low risk and even if unvaccinated/previously uninfected and should not be treated, unless they have significant medical comorbidities, while those who are older or highly comorbid are still at high risk of hospitalization, even with vaccination.
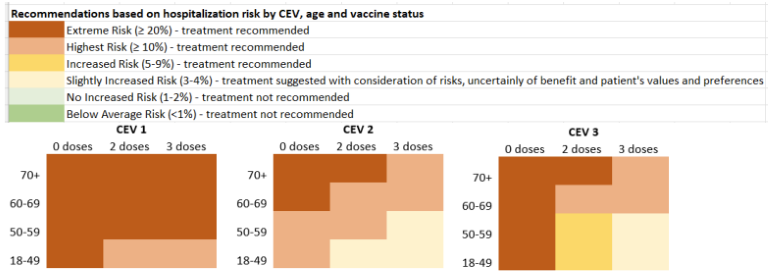
The highest risk are the clinically extremely vulnerable (CEV) subgroups. The CEV-1 group is the most severely immunosuppressed (e.g., bone marrow or solid organ transplant patients), CEV-2 are moderately immunosuppressed (e.g., solid tumors on systemic chemotherapy, biologic agents like TNF-inhibitors), while CEV-3 patients have high-risk comorbidities for severe COVID (e.g., Cystic fibrosis, severe developmental disabilities). For a full list of included conditions see BCCDC CEV Criteria PDF . Although immunosuppressed patients may not respond as well, vaccination is still an important measure. Data on CEV patients admitted for COVID-19 shows similar patterns to other patient populations with older individuals more at risk of severe outcomes, but vaccination provides significant protection against severe disease.
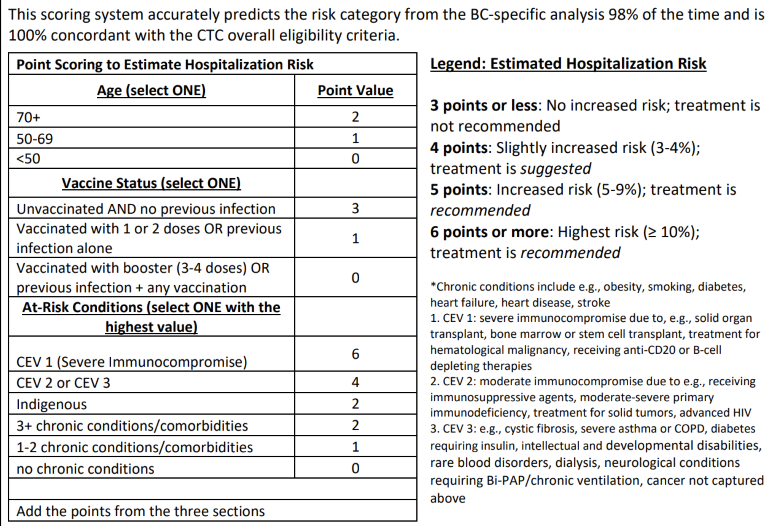
All of these data have been codified into the BCCDC points system that allows risk stratification into low (≤3 points), intermediate (4 points), and high (≥5 points). Nirmatrelvir/rt is currently offered to those at intermediate and high risk of hospitalization (≥4 points). Remdesivir is reserved for those at highest risk of hospitalization who cannot take nirmatrelvir/rt (≥5 points). These data are estimates and do not represent the absolute risk, but rather the relative risk in patients who are identified as cases and seek care.
My approach now is to have the confidence to reassure patients who are low risk that SARS-CoV2 infection will most likely be mild for them and that no specific therapy is needed. Those who are of intermediate risk will also generally do well, but it is an opportunity to create a plan of what to do if they have flu-like symptoms, including their preferences should they test COVID positive. For those who are at high risk, especially CEV-1, I ensure them that there is a clear testing and treatment plan.
With the relatively mild Omicron variant, treatment likely only benefits patients who are symptomatic and not improving after 24-48 hours. If a patient meets the criteria and is not improving, safe prescribing of nirmatrelvir/rt requires some finesse. Common interactions include oral anticoagulants (DOACs – rivaroxaban, apixiban), antiplatelet drugs (Clopidogrel), and statins (atorvastatin, lovastatin). Please see the BCCDC practice tool for a more complete list or an online tool e.g., from the University of Liverpool. Some medications can be held for the 5-day treatment period such as statins, prophylactic clopidogrel, and DOACs for low-risk conditions. For medications that cannot be stopped, it is possible to alter doses or switch to non-interacting medications. For example, dabigatran or low molecular weight heparin injections can replace DOACs. If a patient has a medication that cannot be stopped or modified, discussing IV therapy is the next step, if the patient meets the criteria (≥5 points).
Any physician can prescribe Nirmatrelvir/rt. A step-by-step instruction guide for prescribing is available on the BCCDC website: Assessment Guide for Clinicians PDF. This tool walks clinicians through eligibility criteria, drug-drug interaction management, and how to refer to Health Authority infusion centres for those few patients who qualify for IV Remdesivir. While most prescriptions should be relatively straightforward, there are resources to support you. For complicated drug-drug interactions, there is a pharmacy support line that can be reached at 1-844-915-5005 (more info). For clinical and eligibility questions, support from specialty services such as infectious diseases can be reached through usual consultation routes (e.g. RACE line). For offices where MOAs are triaging calls, a great resource is the COVID self-assessment line (https://covidcheck.gov.bc.ca/) which helps guide patients toward testing and treatment or reassurance.
With nirmatrelvir/rt, a small proportion of patients can have rebound symptoms several days after stopping with rapid antigen testing positivity. The pathophysiology of this phenomenon is unclear. We have no data that suggests a benefit to repeated or prolonged treatment, however, in the absence of data supporting the practice the BC therapeutics committee does not recommend repeat treatment at this time.
Some patients remain very anxious about their risk from COVID and may need reassurance if they test positive. For those who are low risk assure them that COVID usually gets better in a few days and treatment is unlikely to make a difference. For medium risk patients the messaging is more nuanced and involves informing patients that even those of medium risk mostly do very well, but that treatment is possible, depending on their preferences and other health considerations (e.g., interacting medications). For the highest risk patients (e.g., CEV1 patients) those with mild disease that has improved should be reassured, however, those who are not improving quickly should be counseled about treatment options, including IV therapy if oral therapy is contraindicated. For all patients, it is important that they know to seek care should they develop shortness of breath or other worrisome symptoms.
As we move on to COVID being an endemic virus, we will expect to see infections continue. The good news is that we now have the tools to treat patients and prevent hospitalization and death for those at risk. Since the landscape of COVID-19 changes very quickly, keeping up with clinical management is best done by consulting the BCCDC website for health professionals, COVID-19 treatments.
References
- O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature (London). 2021;590(7844):140-145. doi:10.1038/s41586-020-2918-0. (View with CPSBC or UBC)
- Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 386(15):1397-1408. doi:0.1056/NEJMoa2118542. (View)

Great summary. Thank you.
Excellent resource. Thank you.
You do not appear to be considering Long COVID in this article. I am curious what your approach is to monitoring for Long COVID with your patients after their COVID infection and how you are treating this.
Thank you for this helpful summary with many practical suggestions for a rational and patient-centred approach.
Thank you also to the TCMP team and authors for being transparent with disclosures, featuring them prominently at the top of the article! The level of detail and mitigating efforts are useful to know about when considering the content of the article.
Perhaps, more relevant for articles other than this, I always keep in mind that disclosures don’t render a conflict of interest (COI) acceptable, but create transparency which promotes consideration of what is and is not acceptable. Surprisingly, simple disclosure can have the opposite of the intended or expected effect – (see https://www.nytimes.com/2016/07/10/opinion/sunday/the-paradox-of-disclosure.html / Sah S, Fagerlin A, Ubel P. Effect of physician disclosure of specialty bias on patient trust and treatment choice. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(27):7465-7469 for more) – so avoidance of COI is the ideal to strive for.
Keeping up to date on who is at risk, when to consider treatment, and what to do is an ongoing challenge. Thanks for the update.
Makes a lot of sense
UBC has provided excellent webinars and is an excellent resource that I use and willuse the other on :BCSBC as well
Any use of simple OTC meds such as Ceterizine plus Famotidine or a macride antibiotic plus Ceterizine? or a variety of other combinations or an inhaled steroid or Prednisone aimed at limiting inflammation?
We have used this much less expensive approach in our charity work in India and here in BC.
Theee combos are far less expensive and considerably lower side effects and drug interactions.
Problems with paxlovid
Study done on unvaccinated patients
Number needed to treat 34
Very many drug interactions